Project Overview
"The great use of life is to spend it on something that will outlast it." — William James
Aging is the greatest risk factor for most chronic diseases, yet geroscience drug discovery remains largely underexplored.
Guided by the geroscience hypothesis, which posits that targeting the biology of aging can delay or prevent multiple age-related conditions simultaneously, we focus on developing small-molecule gerotherapeutics that modulate key aging hallmarks. Our research integrates interdisciplinary strategies, including drug screening, drug design, medicinal chemistry, and artificial intelligence. In parallel, we investigate the mechanisms underlying gerotherapeutic actions, aging, and longevity using chemical, molecular, cellular, and system-level approaches.
Our goal is to translate discoveries in aging biology into effective interventions that promote healthier, longer lives.
Current projects


Gerotherapeutic Drug Discovery
-
Senotherapeutic Drug Discovery
-
Phenotypic senotherapeutic screening
-
AI/ML-aided discovery of novel senotherapeutics
-
Drug repurposing
-
Developing a cell-based high-throughput senotherapeutic drug screening platform
-
"One-two Punch" Anti-Cancer Therapy
-
Development of Novel SIRT6 Modulators
-
Development of Novel SMAD3 Inhibitors and PROTACs
Aging Biology
-
Ferroptosis as a Senescent Cell Vulnerability
-
Target Identification for Senotherapeutics
Highlighted Projects
Senotherapeutic Drug Discovery
Cellular senescence is a key hallmark of aging and a major contributor to tissue dysfunction and the onset of age-related diseases, including cancer, neurodegeneration, fibrosis, and chronic inflammation.
Senescent cells are now recognized as promising therapeutic targets. Senotherapeutics, which include senolytics (eliminating senescent cells) and senomorphics (modulating the senescence-associated secretory phenotype, SASP), have emerged as a novel approach to extend healthspan and treat multiple age-related conditions.
We employ phenotypic screening, medicinal chemistry, and AI/ML-aided drug discovery to identify and optimize small-molecule senotherapeutics.




Read more
1. Cellular senescence: a key therapeutic target in aging and diseases
Zhang, L.; Pitcher, L. E.; Yousefzadeh, M. J.; Niedernhofer, L. J.; Robbins, P. D.; Zhu, Y.
J. Clin. Invest. 2022, 132(15), e158450.
https://doi.org/10.1172/JCI158450
2. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics
Zhang, L.; Pitcher, L. E.; Prahalad, V.; Niedernhofer, L. J.; Robbins, P. D.
FEBS J. 2023, 290(5), 1362.
https://doi.org/10.1111/febs.16350
3. Recent advances in the discovery of senolytics
Zhang, L.; Pitcher, L. E.; Prahalad, V.; Niedernhofer, L. J.; Robbins, P. D.
Mech. Ageing Dev. 2021, 200, 111587.
https://doi.org/10.1016/j.mad.2021.111587
4. Development of novel flavonoid senolytics through phenotypic drug screening and drug design
Zhang, L. J.;* Salekeen, R.; Soto-Palma, C.; He, Y.; Elsallabi, O.; Hughes, B.; Nunes, A.; Xu, W.; Zhang, B.; Mohamed, A.; McGowan, S. J.; Angelini, L.; O’Kelly, R.; Kamenecka, T. M.; Niedernhofer, L. J.; Robbins, P. D.*
bioRxiv 2024, 2024.10.27.620529.
https://doi.org/10.1101/2024.10.27.620529
5. Identification of lipid senolytics targeting senescent cells through ferroptosis induction
Zhang, L. J.;* Salekeen, R.; Soto-Palma, C.; Elsallabi, O.; Ye, H.; Hughes, B.; Zhang, B.; Nunes, A.; Lee, K.; Xu, W.; Mohamed, A.; Piepgras, E.; McGowan, S. J.; Angelini, L.; O'Kelly, R.; Han, X.; Niedernhofer, L. J.; Robbins, P. D.*
bioRxiv 2024, 2024.10.14.618023.
https://doi.org/10.1101/2024.10.14.618023
6. Fucoidans are senotherapeutics that enhance SIRT6-dependent DNA repair
Zhang, L. J.;* Elsallabi, O.; Soto-Palma, C.; Bartz, J.; Salekeen, R.; Nunes, A.; Xu, W.; Lee, K.; Hughes, B.; Zhang, B.; Mohamed, A.; Mcgowan, S.; Angelini, L.; O'Kelly, R.; Biashad, S. A.; Hillpot, E.; Morandini, F.; Seluanov, A.; Gorbunova, V.; Dong, X.; Niedernhofer, L. J.; Robbins, P. D.*
bioRxiv 2025.04.27.650852.
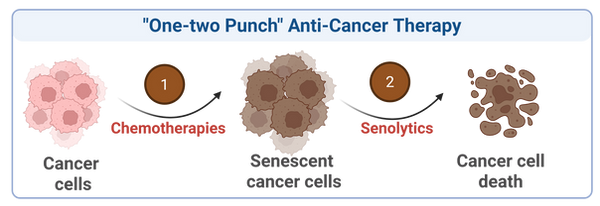
"One-Two Punch" Anti-Cancer Therapy
Conventional cancer treatments, such as chemotherapy and radiotherapy, often induce cellular senescence in cancer cells. These therapy-induced senescent cancer cells can further promote cancer progression, recurrence, metastasis, therapy resistance, and even immunosuppression.
Interestingly, these senescent cancer cells also provide a new vulnerability that can be targeted by senolytics, presenting an alternative therapeutic avenue to improve cancer therapy and reduce chemotherapy resistance.
"One-two punch" therapy, a combination of senescence-inducing agents followed by senolytics, offers a novel dual-action strategy to eliminate tumor cells and improve treatment outcomes.
Our work aims to discover and validate optimal combinations of chemotherapeutics and senolytics to overcome anti-cancer therapy resistance and improve patient outcomes.
Ferroptosis as a Senescent Cell Vulnerability
Our recent work identified conjugated polyunsaturated fatty acids (PUFAs), particularly α-eleostearic acid (α-ESA) and its methyl ester derivative, as a novel class of lipid senolytics that selectively eliminate senescent cells through ferroptosis, a non-apoptotic, iron-dependent form of cell death. These lipid senolytics reduce tissue senescence and extend healthspan in aged mice.
Preliminary mechanistic studies revealed that ACSL4, LPCAT3, and ALOX15 are essential for ESA-induced ferroptosis, establishing a new vulnerability axis in senescent cells. This discovery opens up new opportunities to target senescence through ferroptosis rather than apoptosis-based pathways.
We aim to uncover new molecular vulnerabilities in senescent cells and develop therapeutic strategies that promote healthy aging through ferroptosis induction.
Read more
1. Identification of lipid senolytics targeting senescent cells through ferroptosis induction
Zhang, L. J.;* Salekeen, R.; Soto-Palma, C.; Elsallabi, O.; Ye, H.; Hughes, B.; Zhang, B.; Nunes, A.; Lee, K.; Xu, W.; Mohamed, A.; Piepgras, E.; McGowan, S. J.; Angelini, L.; O'Kelly, R.; Han, X.; Niedernhofer, L. J.; Robbins, P. D.*
bioRxiv 2024, 2024.10.14.618023.
https://doi.org/10.1101/2024.10.14.618023
2. Cellular senescence: a key therapeutic target in aging and diseases
Zhang, L.; Pitcher, L. E.; Yousefzadeh, M. J.; Niedernhofer, L. J.; Robbins, P. D.; Zhu, Y.
J. Clin. Invest. 2022, 132(15), e158450.
https://doi.org/10.1172/JCI158450
3. Recent advances in the discovery of senolytics
Zhang, L.; Pitcher, L. E.; Prahalad, V.; Niedernhofer, L. J.; Robbins, P. D.
Mech. Ageing Dev. 2021, 200, 111587.
https://doi.org/10.1016/j.mad.2021.111587

